Question 92 What is the impact of high iron you have seen in some tight oil feeds? What level of Fe on the equilibrium catalyst causes problems, and what are the typical symptoms? What changes to FCC units hardware, catalyst and operation have you implemented to manage Fe poisoning? What is the impact of other uncommon contaminants such as K, Ca and Mg?
Ann Benoit (Grace Catalysts Technologies)
Tight oil feeds generally have high levels of iron and calcium present in them. Iron can have a negative effect on catalyst performance. While particulate tramp iron from rusting refinery equipment does not have a significant detrimental effect on catalyst, finely dispersed iron particles in feed (either as organic compounds or as colloidal inorganic particles) can deposit on the catalyst surface, reducing its effectiveness. The iron deposits combine with silica, calcium, sodium, and other contaminants to form low melting phases; which collapse the pore structure of the exterior surface, blocking molecules from entering the catalyst particle and reducing conversion. Iron, in combination with calcium and/or sodium, has a greater negative effect on catalyst performance than does iron alone.
The symptoms of iron and calcium poisoning include a loss of buttons cracking and conversion as feed particles are blocked from entering the catalyst particle. In addition to a drop in conversion and a decline in bottoms cracking, poor catalyst circulation could be a symptom of poisoning. A potential indication of Fe poisoning is a drop in Ecat ABD. Nodule formation on the catalyst (shown in figure 1), due to the buildup of Fe on the surface, prevents the Ecat from packing as densely.
Grace has done extensive work on understanding how iron and calcium poisoning impact catalytic performance and has not seen any credible evidence of interparticle iron migration. On the contrary, all the evidence indicates iron poisoning results in a permanent degradation of the catalyst particle surface.

In evaluating the potential for iron poisoning, it is important to calculate the incremental iron from the feed, and not only assess the total iron level on Ecat. This is because different catalysts have different starting iron contents. A general rule of thumb is that performance could suffer with as little as 0.2 wt% incremental Fe, particularly for low alumina catalysts that are more prone to iron poisoning. The level, of course, is dependent upon factors such as the catalyst resistance to Fe poisoning and the concentration of alkali metals contaminants such as calcium and sodium.
To manage iron poisoning, refiners should reformulate to more iron- resistant catalysts and consider higher, fresh catalyst additions. Catalyst design can be optimized to resist the effect of contaminant iron and calcium. High alumina catalyst, especially catalyst with alumina-based binders and matrices, such as Grace’s MIDAS® technology, are best suited to process iron- and calcium-containing feeds because they are more resistant to the formation of low-melting point phases that permanently destroy the surface pore structure. Optimum distribution of mesoporosity (pores in the 100--600Å size range) also plays a role in maintaining performance because diffusion to active sites remains unhindered, even with high levels of contaminant metals. One thing to consider, while two catalysts may have similar total pore volume, their mesoporosity can vary greatly. MIDAS® catalysts feature the highest levels of mesoporosity in the market.
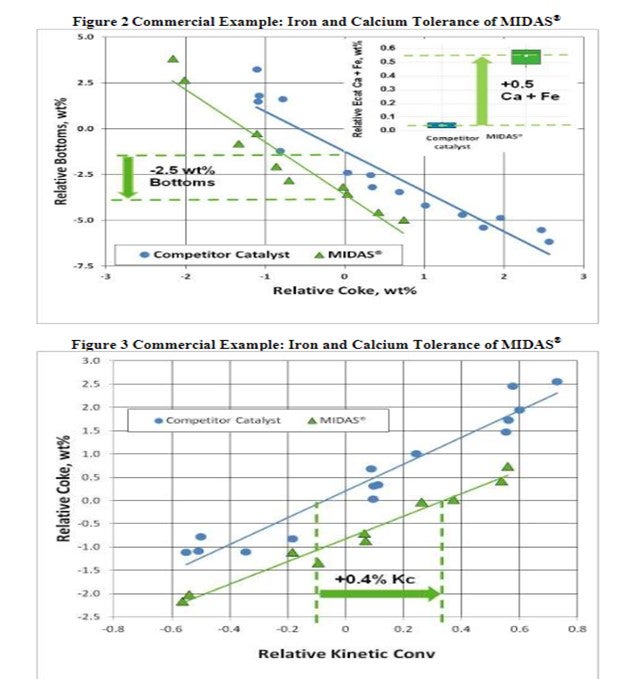
The resistance of MIDAS® to iron and calcium poisoning was demonstrated in a commercial application (Figure 2 and Figure 3). A refinery was processing a feedstock high in iron and calcium. Over time, the unit exhibited the symptoms of iron poisoning. Iron nodules built up on the catalyst surface; equilibrium catalyst activity, unit conversion and bottoms cracking began to suffer. The catalyst was switched from a competitive catalyst to MIDAS®. Upon switching, activity, bottoms cracking, and coke selectivity improved despite the higher metal's levels.
With regard to other uncommon containments, alkali metals such as potassium can result in deactivation of the FCC catalyst, particularly under the oxidizing, high temperature conditions in the regenerator.
Alkali metals cause a loss in activity due to neutralization of acid sites. The result can be a loss of unit conversion. On a weight percentage on equilibrium catalyst basis, the deactivation effect of potassium is similar to that of sodium.5 Magnesium is not of concern at low levels (<0.5 wt%), but at higher levels, magnesium has a tendency to react with the silica from the zeolite to form forsterite (Mg2SiO4), which will decrease zeolite stability and adversely affect unit conversion.6 As mentioned above, calcium poisoning can be a serious problem, reducing bottoms cracking and catalyst activity. Like Fe, Ca deposits on the exterior surface of the catalyst,2 as calcium builds up on the surface, the particle becomes compromised, and this can result in unit conversion loss. In conclusion, catalyst reformulation (to more resistant formulas such as Midas®) and higher fresh catalyst additions to purge the contaminants from the inventory are effective strategies to recover from non-conventional contamination in the FCC unit.