Question 69: How do you detect that amine salts are forming and causing corrosion, either in the fractionator or other locations ahead of the water dew point? What are chemical and operational strategies for mitigation?
LEE (BP North America)
We have the chemical service vendor perform routine calculations for ammonia and/or amine salt points. We typically monitor the water dew point ourselves. The amine salt point is to be differentiated from the ammonia chloride salt point. Depending on the amine, a salt point can be higher, and often is. The amine, as opposed to the ammonia salt point, calculation is typically by special request if there are issues with the particular unit operating near the system’s salt point. It should be noted that data requirements for doing an in-tower amine salt point are more difficult and onerous than for doing an amine salt point in the cool tower overhead system for which the amine content in the sour water is readily available by direct analysis. The calculation for the in-tower amine salt point requires an estimate of the amine quantities feeding the crude tower and prior to any overhead chemical injections.

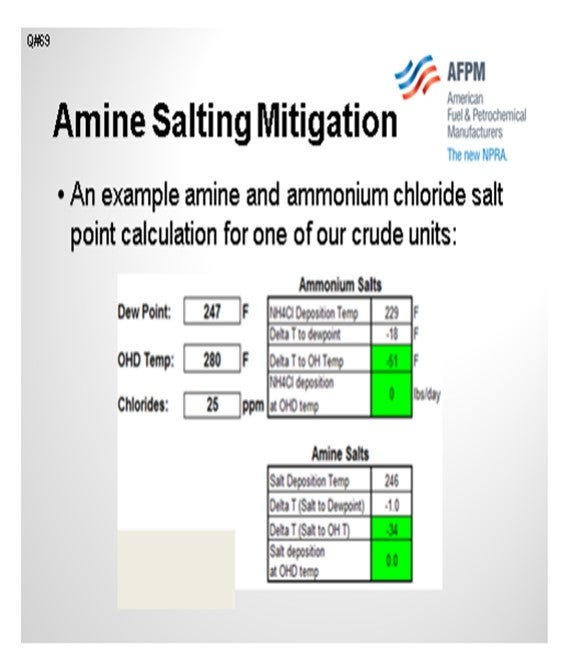
The table on the next slide shows some amine salt points for one of our crude tower overheads and compares them to the ammonium chloride salt point. In this example, the ammonium chloride is in the 229°F range, and the amine salt is 246°F in the crude tower overhead system. We try to maintain the top pumparound return temperature above the controlling salt point. We also try to operate the bulk top temperature at least 40°F above the controlling salt point. But recently, there has been a tendency with commercial drivers to reduce top temperature targets in order to maximize jet or light diesel production over naphtha production.
If the system is in a troubleshooting mode and corrosion is suspected, then samples are taken from any deposits in the top pumparound circuit and analyzed for amine, nitrogen, and chloride content. Of course, if corrosion products are present, they will be evident by significant iron and other inorganic content in the foulant samples.
Some other mitigation handles on amine salt formation include the following:
1. Optimize desalter performance to reduce hydrolyzed chloride content in the crude tower overhead. There are multitudes of desalter optimization parameters, including washwater rate, washwater quality enhancements (including pH and ammonia content), mixed valve pressure drop optimization, demulsifier use, and optimization of grid voltage settings to achieve the maximum operating temperature.
2. Use caustic injection as an operating handle if caustic injection skids and the injection quill are already available on the unit. About half of our units practice caustic injection to mitigate the chloride’s contribution to amine salt formation.
3. Review filmer or neutralizer formulation with the chemical service provider. Certain filmers and neutralizers have slightly lower amine salt point than others, and there are tradeoffs in performance.
4. Operate the top temperature of the crude tower or elevated temperature, which will increase the operating margins of the salt point. This is in commercial conflict with the typical current financial drivers to minimize overhead naphtha production while increasing jet or kerosene. There will be short-term yield optimization versus long-term availability tradeoffs.
5. If possible, increase the top pumparound return temperature. Again, the idea here is to increase operating margins to the system salt point while trying to maintain the required crude tower heat removal.
6. Perform continuous washwater injection at the location of salt deposition.
DION (GE Water & Process Technologies)
As previously discussed, the ionic equilibrium model is an extremely important tool to define the safe operating envelope for your unit. Most specialty chemical suppliers have an ionic equilibrium model. GE’s ionic equilibrium model is called LoSalt*. I cannot stress enough the importance of running these models as frequently as possible. Various scenarios can be examined in advance to predict when particular parameters would increase the risk for salt corrosion. Different neutralizing amines will have different salt points. Selecting a low salting amine will minimize salt corrosion potential. Then explore the other parameters, such as the upper limit for tramp amines, chlorides, and stripping steam. The best-in-class corrosion control program will look at all of these factors: metallurgy, mapping and eliminating tramp amines, overhead waterwash, an effective filmer, and the appropriate neutralizing amine.
DEAN WONG (Husky Energy)
We have heard a lot of good information about desalting operations, but does the panel have experience removing crystalline salts as the feed gets heavier, the easy salts?
DION (GE Water & Process Technologies)
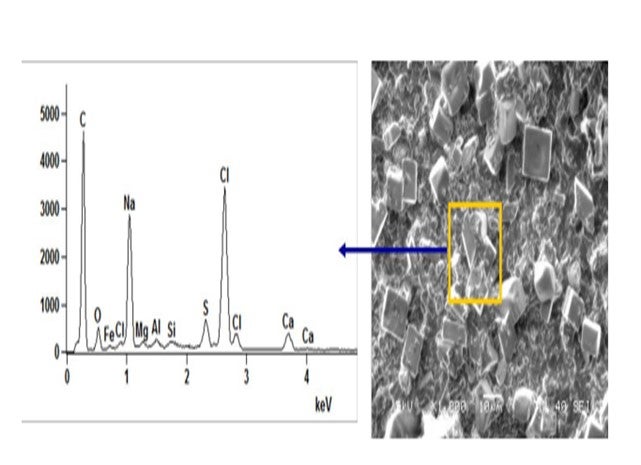
Crystalline salts exist in crude. Below is a microscopic picture and analysis of raw crude after filtration. The topic of “encapsulated” or “asphaltene-coated” salts has been mentioned in the past. In general, their removal can be improved by working the desalter harder. The crystalline salts must contact the washwater to be removed from crude. The desalter is designed to make an emulsion, wash impurities from the crude, and then resolve the emulsion. A finer emulsion, using mix valve and washwater rates, can be utilized to improve salt removal efficiency. The Salt by Extraction Test should measure all extractable chlorides. The procedure includes adding hot toluene to the crude in a separatory funnel. In theory, the toluene should remove the heavy oil and asphaltene coating from the crystalline salts.
RUSSELL STRONG (Champion Technologies)
I love this topic, but I will not take too much time. Let’s go back. The question says, “How do you detect if amine salts are forming and causing corrosion?” I will read that as “…have formed and are causing corrosion”. A main consideration has to do with neutralizer injection. In the case of a neutralizer, if it goes in as a liquid, which is typical, then it will take time for it to absorb the latent heat. The neutralizer will need to evaporate in order to work according to modeling. In the course of that evaporation, it will have ample opportunity, while as a liquid, to react with HCl vapor. That problem is seen in a lot of refineries, particularly if they do not have the right kind of injection system to inject the neutralizer as a vapor.
In some cases, even if you think you have a good injection system, you may still have a similar problem. A high concentration of salt can form, caused by amine recycling to a higher and higher concentration. Modeling may not have adequately considered the amount of neutralizer partitioning into reflux and/or into the desalter crude, which then cycles back to the overhead.
All that being said, let’s get to the question of detection. You can usually tell if salts have formed ahead of the water dew point. Pull out a corrosion probe/coupon from upstream of the calculated water dew point and examine it using EDS-SEM (energy dispersive spectroscopy-scanning electron microscopy) analysis. You can see any chloride that may be on that probe/coupon. The chloride will have been deposited in a salt form. HCl vapor is certainly not going to condense ahead of the water dew point.
The trouble with knowing whether or not it is an amine chloride, ammonium chloride, or some other form of chloride is that when corrosion occurs, the salt forms metal-chloride. The amine/ammonia has nothing to hold it in place. It tends to evaporate away from the deposit, and subsequent examination will show significantly less amine than was originally present. Alternatively, if you cannot do the above analyses, a chloride salt on your corrosion probe, on which you had an amine salt or an ammonium chloride salt, tends to top out around 50 mils a year unless there is a velocity-assisted component that accelerates the corrosion. If it is less than that, the problem is not as serious as it could be.
DION (GE Water & Process Technologies)
A more complete understanding of the overhead system dynamics is the first step in properly diagnosing the corrosion and fouling potential of a given unit. In the past, this has been accomplished retroactively following the failure of system equipment. However, a proactive approach to amine salt precipitation prediction and corrosion mitigation is a much more efficient way of controlling overhead corrosion. A highly effective means of determining the formation of amine salts is through the use of ionic modeling software. The use of a robust and rapid ionic modeling tool will allow for the calculation of the salt precipitation temperature, or “salt point”, of the neutralization salts and water dew point (ICP) temperature. The use of such a model allows for rapid evaluation of current system variables and operating parameters. With this information, asset operators can potentially adjust tower operating conditions (e.g., overhead outlet temperatures) to manipulate the location in the overhead system where the neutralization salts precipitate. Use of the model also allows for the development of an “operating envelope” for the tower and overhead system that keep the amine salts from forming in unwanted areas of the overhead circuit across various desalter effluent chloride ranges. GE has successfully implemented this control and mitigation approach at a client site using its LoSALT* Ionic Modeling Tool and the refiner first consults with the local representative to evaluate potential tower operational changes prior to adjusting process conditions.
Beyond operational adjustments, a “three-prong” approach of neutralizer, waterwash, and filming corrosion inhibitor should be employed to mitigate corrosion by amine salts. First, an appropriate neutralizer must be selected to minimize the neutralization salt precipitation temperature while effectively controlling ICP and accumulator pH within acceptable ranges. Lower salt-point amines should be utilized in the overhead treatment program to minimize salt formation in the overhead system ahead of the water dew point or in the fractionator tower itself. Secondly, an effective waterwash protocol must be implemented to raise the amount of free water in the system and provide a means of washing away any precipitated amine salt deposits. The last portion of this chemical treatment approach is to introduce a quality filming corrosion inhibitor to protect the metal surfaces against corrosive attack. Some quality filmers will provide a level of salt dispersions to further aid in the movement of amine salts through the system and reduce underdeposit corrosion related to these salts.
Tramp amines that enter the distillation column with the desalted crude contribute to higher salt point temperatures in the overhead. An often-overlooked operational strategy is source-reduction of the tramp amines that would drive the overhead salt point to higher temperatures. Developing an amine-map of the refinery’s amine sources can aid in rapidly identifying and eliminating tramp amines entering the overhead system. Additionally, chemical treatments can be implemented at the desalter to increase amine extraction into the desalter effluent brine and prevent them from reaching the tower overhead.
BASHAM (Marathon Petroleum Corporation)
Ionic modeling is the only way to predict. High pressure drops across the top couple of trays could point to a buildup of fouling material from corrosion. The top temperature of the crude tower should stay above 300°F to ensure that no corrosion happens in the tower. Consideration should be given to ensuring that the top pumparound return temperature is well above the water dew point temperature to avoid shock condensation of water vapor. Also, reflux should be introduced onto the top tray with a spray header to allow it to heat up and not cause shock condensation.
RANDY RECHTIEN (Baker Hughes)
The best method for determining the formation potential of amine-HCl salts is the use of Baker Hughes Ionic Model technique. This method provides for the rigorous calculation of salt formation temperatures under all operating scenarios. Once the root cause of the salt formation has been determined, multiple mitigation options can be examined using the Ionic Model. These mitigation options include the following:
• Improved desalting operation to reduce overhead HCl levels,
• Caustic injection to desalted crude to reduce overhead HCl levels,
• Application of alternative neutralizers with lower salt-forming tendency,
• Installation of continuous waterwash to the overhead, and
• Increased tower operating temperatures.
DENNIS HAYNES (Nalco Energy Services)
Salt formation in towers would result in restricted flow and reduced distillation performance. Where salt formation is towers is suspect, as tower scan would assist in determining location and severity. Overhead lines that are suspect for salt formation should have UT reading periodically done and changes in corrosion rates would be an indication. Both of these points are after-the-fact measurements; so more importantly, utilization of modeling to determine salt points in process towers would lead to an early indication of potential problems.
When salt has formed and needs to be dealt with salt dispersant chemistries have an effective history in the industry. Operationally, slumping the tower and applying a waterwash is possible; however, much planning and care is required due the introduction of water into a potentially hot system. If salt formation is possible, washwater, additional steam, adjusting tower temperature, and pressure are all variables that may be used to change dew point and salt points.