Question 61: What are some of the potential strategies to mitigate iron carryover from the desalter?
HODGES (Athlon Solutions)
High iron content on desalted crude manifests itself in two main areas. These are inability to make anode-grade coke and as a FCC catalyst poison. The iron can be in many forms. Three of the most common forms of iron we see are iron oxide, which is rust; iron sulfide, which is corrosion; and, siderite, which is essentially crystalline iron carbonate that we are seeing more and more of in certain tight oils. Historically, operators and process chemical suppliers have tried to address iron removal in three ways. The first way we have seen is acidifying the washwater with citric, acidic, or glycolic acids. A second way is applying a chelant to the washwater such as EDTA (ethylenediaminetetraacetic acid). The third way is running a continuous dirty rag draw from the desalter. We have not seen any of these mentioned methods meet with much success with respect to iron removal, and they have the unpleasant side effect of increasing desalter, crude tower, and downstream unit corrosion. The handling problems of the continuous dirty rag have diminished the popularity of that method as well.

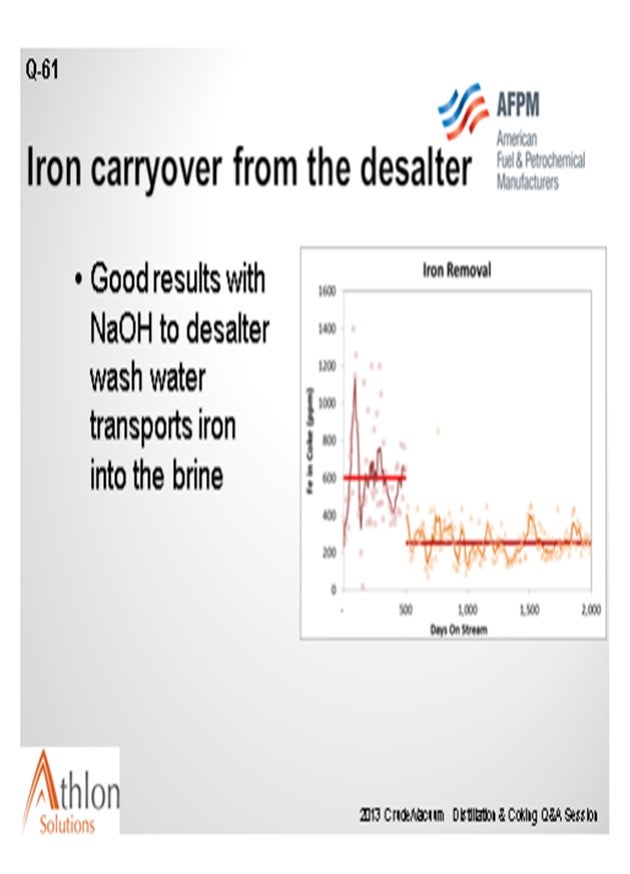
We have seen a very successful alternative approach where caustic is added to the desalter washwater along with a unique demulsifier, which functions well at all pH levels. This transports the iron into the water phase, thus exiting with the brine. Check with your current chemical desalter supplier before trying this approach.
JIM PROROK (Husky Oil Operations Ltd.)
For years, we have been successfully running the dirty bleed pull on the rag draw, and we continue to do so. Yes, we are an anode coke business, and good iron removal is essential. Maybe the difference is that they were a light sweet refinery. Nowadays, the solids contents are coming up in the crudes, but we still manage them. You are right; we must have it. The oily bleed has to go to a free water knockout drum and then get as much free water out as possible. The resulting oil stream has to be centrifuged.
HODGES (Athlon Solutions)
It can be managed; it is just a bit cumbersome.
SLOLEY (CH2M Hill)
What pH do you run the desalter water down when you add caustic for the iron partitioning in order to promote iron partitioning into the water?
HODGES (Athlon Solutions)
The pH of the effluent brine must be 9 or higher. You could actually do some studies of pH elevation versus your iron removal to determine the specific best target for your particular desalter.
ROBERT AJILUNI (Athlon Solutions)
I want to add that if the source of your washwater is from a sour water stripper, you will need make sure that the stripper is working well because sometimes that water will have an elevated pH due to ammonia. So, if you are checking your washwater or brine as it comes off the desalter, you would think: Oh, the pH is good, and I have plenty of caustic. But in fact, you do have not enough caustic; so, you are not actually removing the iron the way you should. It is such a fine line on the color of that brine. You do not want crystal-clear brine, but you do not want oil; so that it is really incumbent on the operator, and Operations in general, to walk that fine line. But if you walk that line, you can get some really amazing results.
JILL BROWN BURNS (Valero Energy Corporation)
We actually do this at one of our facilities and have not really seen the benefits on most of our critical KPIs (key performance indicator). It has actually caused some difficulties. If you go back to one of our previous slides talking about controlling the crude overhead pH, you will see that this is the KPI we are not able to meet. Mr. Sloley indicated controlling the brine pH. It does go up when you add caustic to it, and then you see a subsequent issue with controlling the crude overhead pH below the upper specification.
GLENN SCATTERGOOD (Nalco Champion Energy Services)
Some contaminant removal additives (CRAs) can be added to the desalter washwater to make the iron more water-soluble (such as acids) and move it from the oil phase into the water, leaving the desalter, along with the brine water, to the wastewater treatment plant in a water-soluble form. Other CRAs are non-acidic and can be used to move particulate iron from emulsion layers in the desalter into the water phase.
CHRIS CLAESEN (Nalco Champion Energy Services)
If the largest part of the Fe is contained in the solids, the solids removal can be improved with a specific Nalco Champion solids wetting additive. Removal of over 90% of the Fe and desalted crude Fe levels below 0.5 ppm have both been achieved with this additive.
DENNIS HAYNES (Nalco Champion Energy Services)
Mitigation of iron carryover from the desalter depends on the form of the iron; but where it is iron sulfide particulate, increased washwater, optimized mix valve setting, addition of solids removal chemistries, and adjusted interface level are the most certain methods.
PHILIP THORNTHWAITE (Nalco Champion Energy Services)
The key consideration here is the form of the Fe. In the majority of cases, iron is present as iron sulfide, a corrosion byproduct which is present in the crude due to corrosion upstream, tankage, or introduced via external streams such as slops. The particle size of the FeS (iron sulfide) in the crude is very small; and when coated in oil, these particles are very difficult to remove at the desalter. In order to effectively removed inorganic iron present as particulates, specialist solids removal chemistries have to be used in conjunction with the primary demulsifier. When the oil and washwater pass through the mix valve, the adjunct products help remove the oil coating from the particulate, allowing it to be water wetted. Once water-wet, the solids are removed with the effluent brine.
Iron can also be in the form of iron naphthenate and is present in particular naphthenic acid crudes. This organic form of iron is effectively removed through acidification of the washwater. The reduced pH causes the iron to dissociate from the naphthenate molecule, leaving the naphthenic acid and the iron salt of the particular acid that was used. This iron salt is typically water-soluble and removed with the effluent brine.