Question 34: Fresh sulfuric acid fed to an alkylation unit can contain niter (nitrosylsulfuric acid) which may lead to excessive corrosion. What is niter; what does it do; can we test for it; and how can we reduce the levels in our fresh acid?
MUEHLBAUER (Valero Energy Corporation – Benicia Refinery)
“Niter” is actually a common term referring to the amount of NOx (nitrogen oxide) or nitrates in the sulfuric acid. It is actually generated in the sulfuric acid regeneration process. One of the first steps you go through in acid regeneration is a combustion furnace. In that combustion furnace, you can generate NOx, which is really a function of your peak flame temperature and excess oxygen: the normal NOx contributors. But then, what the regeneration facility does with
that NOx is what can cause a variation in the niter concentrations. Some units are designed with a mist break eliminator that essentially can entrain this niter in the product fresh sulfuric acid, in which case the concentration in that stream would be much higher.
Other facilities either employ different technology for NOx control, or they are able to segregate the niter and reroute it to the spent acid. The niter stream becomes a feed to the regeneration unit, which would then get reprocessed and not end up in the product fresh acid. Within Valero, we actually have not had much experience with niter-related corrosion, nor have we had any of our corrosion caused by niter. Our acid supplier told me that our niter concentration typically ranges from 25 ppm to 50 ppm levels.

PIZZINI (Phillips 66)
I want to give a nod to Randy Peterson who provided a lot of the information for this response. As Joe said, niter is the NOx compound that can exist in the finished sulfuric acid. There are wet chemistry methods that can quantitatively measure how much niter is present. One method is a color change test with iron sulfate. I know that DuPont and Rhodia both have methods. You need to get in touch with either of those companies to get the method. The amount of NOx is influenced by how hot the acid regen furnace is operating. So, if you are burning just all-natural gas for heat, this high temperature flame will increase the NOx versus, say, a spent acid furnace that is burning acid gas. We have input indicating that the typical niter levels from a two-stage spent acid furnace are 25 ppm to 40 ppm.
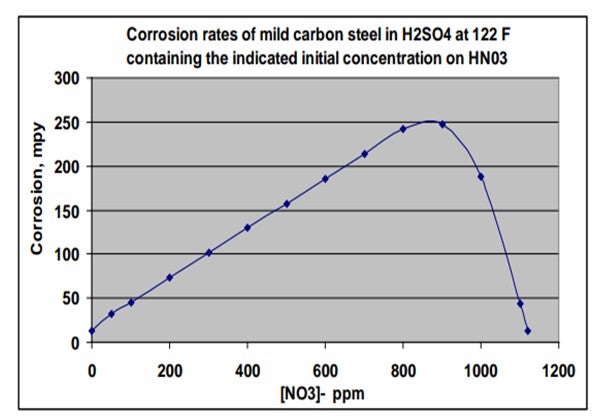
The chart on this slide was created based on an equation included in an article in a 1980 metallurgical trade journal. I plotted out the information to show the corrosion rate of carbon steel in mils per year, based on a scale of 0 to 300, relative to how much nitrate is present in strong sulfuric acid at 122ºF. So, if you get upwards of 800 ppm of nitrates, corrosion could be very aggressive. The good news is that the NOx is consumed in that reaction, so it is not self0-sustaining and does eventually disappear in a closed system. If you get above 1,000 ppm of niter in acid, then the corrosion rate will actually drop as a result of having a passivation effect.
PIZZINI (Phillips 66)
“Niter” is a term that describes the amount of dissolved nitrogen oxides in sulfuric acid. The term is applied to the amount of either NO3 (nitrate) or NO2- (nitrite) present. This is a relevant question, because increased levels of niter will affect the corrosion rate of the acid on carbon steel since these compounds attack the protective ferrous sulfate coating on the metal surface. There are “wet-chemistry” methods to determine the amount of niter in sulfuric acid, available from DuPont and Rhodia. This test uses ferrous sulfate which reacts with nitrates and nitrites to produce a red color. The amount of niter in sulfuric acid is related to the conditions in the spent acid regeneration furnace, where hotter flames (e.g., burning only natural gas) or the presence of ammonia in the fuel gas will lead to higher NOx levels in the resulting fresh acid. For example, a two-stage spent acid furnace would be expected to have niter levels in the 25 ppm to 40 ppm range in the fresh acid.
Corrosion rate effects on mild carbon steel in concentrated sulfuric acid were documented in a 1980 AIME (American Institute of Mechanical Engineers) trade magazine, Metallurgical Transactions A, Volume 11A. The chart below was creating using information from that article. Note that the corrosion rate increases with nitrate concentrations up to approximately 1,000 ppm, and then decreased markedly at higher nitrate concentrations, where the steel passivity is re-established. Also note that the nitrates and nitrites are reduced (converts to NO and N2O gasses) as they accelerate corrosion and are therefore self-limiting in closed systems.
RANDY PETERSON (STRATCO® - DuPont)
We have recently seen excessive corrosion problems in sulfuric acid alkylation units that are being run very close to optimum design conditions. After significant trouble shooting and testing for many contaminants, we believe that unit corrosion is significantly accelerated by a contaminant found in all fresh sulfuric acid. This contaminant is nicknamed “niter” and is mostly composed of nitrosylsulfuric acid (NSA). Its chemical formula is NOHSO4 and it acts as an
oxidizer that apparently is very corrosive to carbon steel and Alloy 20. Niter is formed in the sulfuric acid regeneration plant (SAR) and is most concentrated in the drains from the mist eliminators within the absorption towers (i.e., final absorption and interpass absorption towers). These drains can either be allowed to drip into the tower bottoms where the niter combines with the product acid or can be collected within the towers and routed to another destination. This destination is typically either the 99 wt.% product acid tanks, the 90 wt% spent acid tank (SAR feed) or directly into the SAR furnace.
We have found that if the niter-rich drains are sent to the product acid, the niter content in the fresh acid sent to the alkylation unit can be as high as 300 ppm. If the drains are sent to the spent acid tank or directly to the furnace, most of the niter is converted to N2 within the SAR and harmlessly exits the stack with the flue gas. The product acid then typically contains less than 30 ppm niter. We suggest that you contact your sulfuric acid supplier and ask them, “Where are you sending the niter-rich drains from the mist eliminators?” Of course, we recommend that they not be added to the fresh acid that you purchase. If you have your own onsite SAR and the drains are not routed to the furnace or spent acid tank, we recommend that you contact your SAR licensor for Best Practice recommendations. If you would like DuPont’s colorimetric test procedure for measuring niter in fresh acid, please email me at: j-randall.peterson@dupont.com.
Here are a few pictures of what we believe to be niter-induced corrosion. The first picture is of Alloy 20 Acid Wash coalescing media that had gone “active” and collapsed within two years. This is typically considered a very mild service, liquid-full, with mostly isobutane, some alkylate, and less than 5 LV% of 99 wt.% acid. The temperature is approximately 85°F and the velocity is extremely low (<10 ft/min (feet per minute). The Chevron-type Alloy 20 coalescing
media originally filled the entire area between the wire grids. Notice that the carbon steel vessel and the 316 SS (stainless steel) perforated plate and support grids appear to still be in very good condition. Alloy 20 is a specialty metal that is specifically formulated for harsh sulfuric acid services. The failed Alloy 20 was analyzed by DuPont metallurgists and found to have the proper composition.

The following pictures are samples cut from a prematurely failed carbon steel Contactor™ reactor tube bundle. These tubes failed in less than two years under what is typically considered mild conditions (<50°F and 92 wt% acid). Pitting is not a typical failure mechanism of carbon steel in sulfuric acid service. This is the primary clue that led us to look for the presence of oxidizers such as niter.

