Question 2: We have a Selective Hydrogeneration Units (SHU) unit that removes butadiene from the olefin feed to the alkylation unit. What is the typical concentration of hydrogen and light ends in the olefin product leaving the SHU? How do hydrogen and light ends affect alky operations?
THOMAS PORRITT (Chevron U.S.A)
A selective hydrogenation unit or SHU converts diolefins to olefins in a stream before it feeds an alkylation unit.
Below is a basic flow scheme.
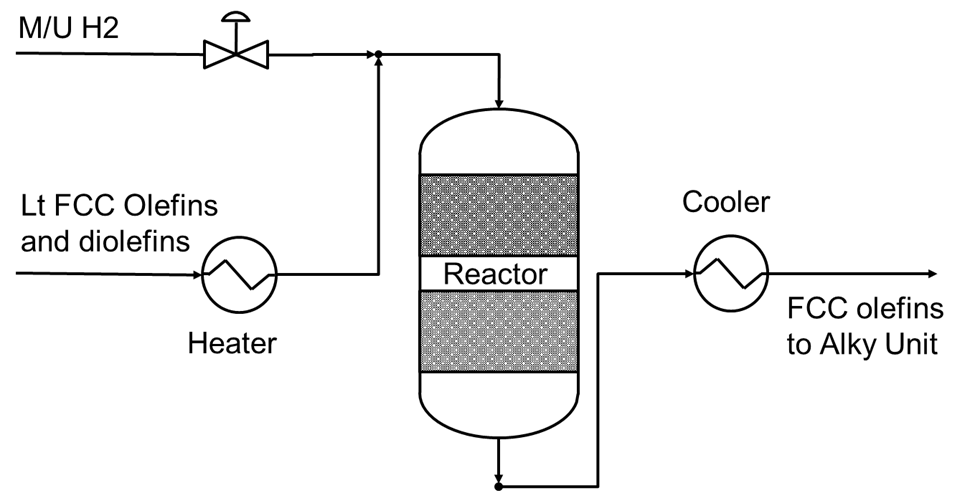
Figure Q2 - 1 Selective Hydrogenation Unit Flow Scheme
The typical composition of non-condensable gases and light hydrocarbons leaving the SHU is dependent upon both the feed and the hydrogen make up. The olefin feed may contain methane, ethane and ethylene in addition to the olefins and diolefins that pass through the reactor. The hydrogen composition can contain a mixture of light hydrocarbons as well as carbon monoxide and carbon dioxide depending on the source. A component balance on these contaminants can show their concentrations in the SHU effluent for the user’s system.
Hydrogen sources vary by nature and quality. Hydrogen produced by a Steam Methane Reformer, SMR, with a pressure swing adsorption, PSA, unit will be the cleanest. PSA units can produce hydrogen that is greater than 99% pure. Not all SMR hydrogen is purified by a PSA. For SMR hydrogen that passes through methanation and chemical absorption, the hydrogen can contain methane and possibly carbon dioxide. At the other end of the spectrum is naphtha reformer hydrogen. This hydrogen can be as low as 70% hydrogen by volume. Reformer hydrogen can contain as much as 8% methane and 6% ethane by volume. The amount of carbon monoxide is dependent on the system pressure of the naphtha reformer. Low-pressure units can produce hydrogen with as much as 30 ppmv carbon monoxide. Excess hydrogen in the SHU leads to oversaturation of the olefins feeding the alkylation unit. In addition, it leads to increased reactor differential temperature and can potentially damage the palladium catalyst in the reactor beds.
The light hydrocarbons and non-condensable gases exiting the SHU pass to the alkylation unit. They then build up and lead to increased pressure in the refrigeration loop. Because they do not condense, they reduce the capacity of the refrigerant compressor and condenser. To manage pressure, the user is forced to vent the non-condensable gases at the refrigerant accumulator. Unfortunately, this venting also leads to loss of iso-butane.
To mitigate these issues the user can proactively clean up the light hydrocarbons in the FCC olefins and better purify the makeup hydrogen. In addition, the user can adjust the hydrogen to olefin ratio by improving control of hydrogen to the SHU. As a final step, the user can vent the gasses at the refrigerant accumulator.
McDermott-Lummus Technology and Dupont Clean Technologies both contributed to this answer.
BILL KOSTKA (Axens North America)
The unreacted hydrogen in the effluent of a Selective Hydrogenation Reactor is very low, typically within the range of 20-50 ppmw.
Methane in the effluent of a Selective Hydrogenation Reactor comes directly from the hydrogen make-up and thus depends on its quality. For PSA hydrogen, the typical amount of residual methane in the reactor effluent is less than 10 ppmw. For a hydrogen-rich stream containing 90 mole% hydrogen and 10 mole% methane, the methane content rises up to typically 500-1,000 ppmw in the reactor effluent.
In a Sulfuric Acid Alkylation unit, ethane and lighter may cause venting from the refrigeration receiver if levels are high. The vent would carry some isobutane, but no sulfuric acid, and would typically go to the flare. A small, packed scrubber may be needed to remove SO2 if venting is done on a continuous basis. Pentanes in the feed cause no operational problems since they will end up in the alkylate product. However, they could result in difficulty meeting RVP and octane targets if these are critical.
RICK DENNE (Norton Engineering Consultants, Inc.)
A typical design would include a downstream stripper or de-ethanizer column to remove excess hydrogen and light ends to the plant fuel gas system. The column would operate in such a manner that propylene/propane loss are minimized, so as to not downgrade their value. Ethane and lighter streams would be rejected. If fractionation is poor, the stream could be routed to the plant’s sat gas unit; however, care must be taken to avoid putting the liquid propane product off test for excessive propylene. If a stripper or de-ethanizer column is absent, the light ends will act as a diluent in the alkylation unit and the non-condensables will cause pressure issues in various parts of the unit. In this case, the owner/operator should weigh the option of minimizing hydrogen to the SHU, at the expense of more rapid catalyst deactivation, versus alkylation unit limits. Improved make-up hydrogen quality should also be considered.